Effect of Molecular Weight
on Polymer Properties
Almost all properties of a polymer depend on the molecular weight and its distribution, that is, low and high molecular weight polymers will have vastly different mechanical and thermo-physical properties. For example, polymeric materials consisting of a rather small number of repeat units (often called oligomers1) will be soft or rubbery solids or low viscous liquids and will possess little or no strength whereas polymeric materials of high molecular weight will be solid, and have much improved mechanical properties. One usually observes a steep rise in the mechanical and visco-elastic properties with increasing molecular weight until a certain molecular weight is reached, beyond which the properties are nearly independent of the chain length, that is, all properties will eventually reach an asymptotic value. It was found, that the molecular weight dependence of several thermodynamical properties can be described by an expression of the form2-4
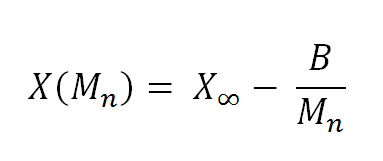
where X is a certain thermodynamical property, X∞ is its asymptotic value at infinitely high molecular weight, and B is a constant.
Some polymer properties that are very sensitive to the molecular weight include softening and melting point, glass transition temperature, solution and melt viscosity, solubility, mechanical strength and moduli.
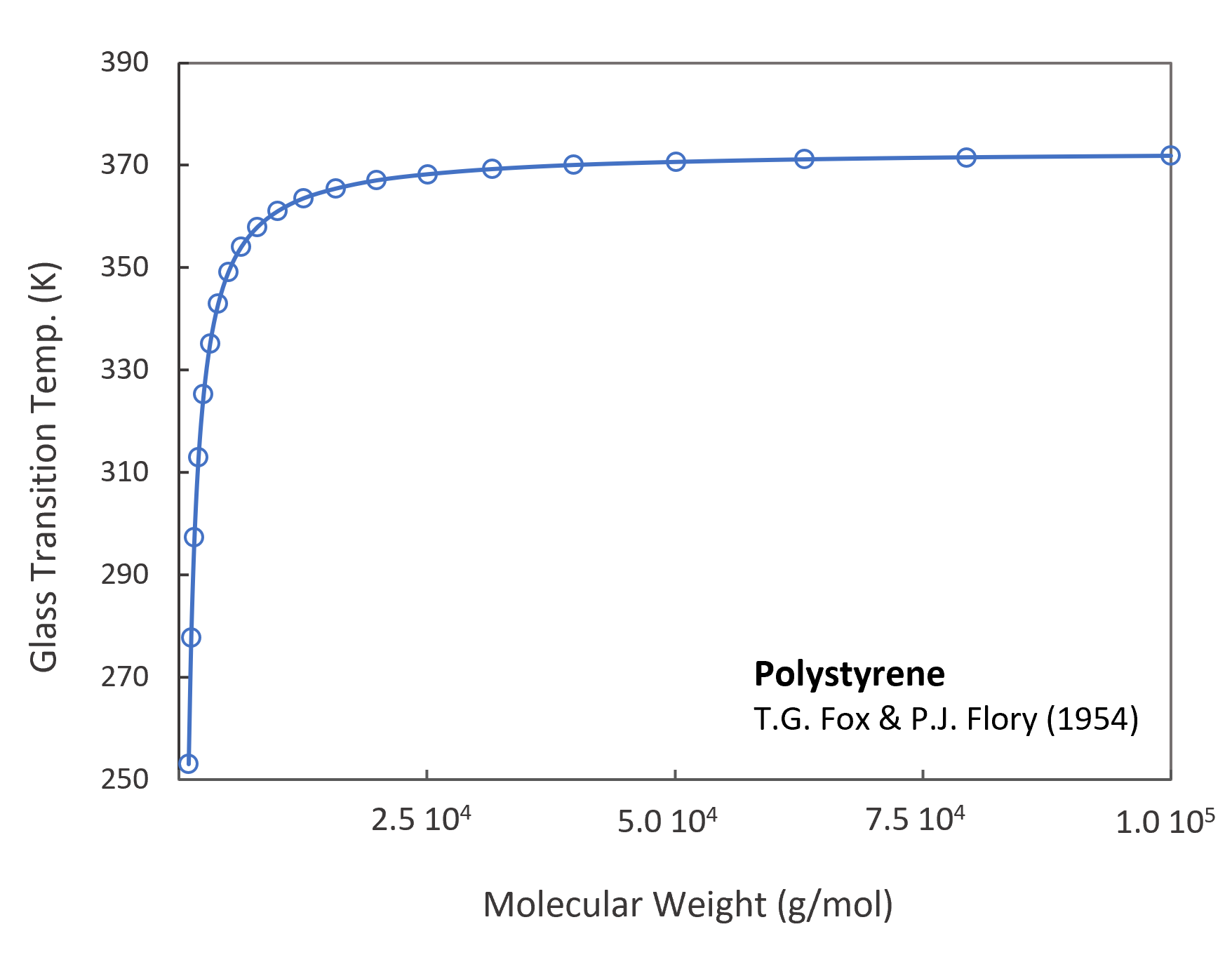
As an example, in the figure above the glass transition temperature (Tg) as a function of molecular weight is plotted for polystyrene. The Tg values have been predicted with the Flory-Fox equation:5,6
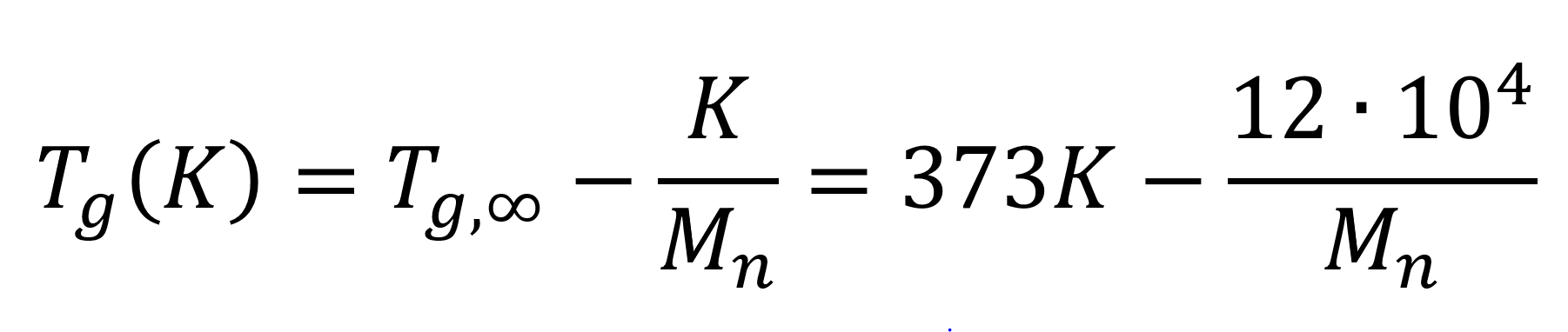
where Tg,∞ is the asymptotic glass transition temperature at very high molecular weight.
Not only the average molecular weight but also its distribution has a noticeable effect on the physical, mechanical, and processing properties. In most cases, a narrow molecular weight distribution will yield better mechanical properties than a broad distribution because the low molecular weight portion will act as a plasticizer and soften the polymeric material, whereas an exceedingly high molecular weight portion will make processing of the polymer resin very difficult due to its disproportionately high contribution to the melt viscosity.
References and Notes
Oligomers have properties in between monomers and polymers. Since they are made up of a relative small number of repeat units, reomoval of one unit has a noticeable effect on their properties which is not the case with polymers. Typical examples of oligomers include liquid paraffin, curable resins such as epoxides and phenolics, as well as many plasticizers, tackifiers and lubricants.
- D.W. van Krevelen and K. Nijenhuis, Properties of Polymers, 4th Edition, Amsterdam (2009)
- L. H. Sperling, Introduction to Physical Polymer Science, 4th. Ed., Wiley & Sons 2006
K. Balani, V. Verma, A. Agarwal, R. Narayan; Physical, Thermal, and Mechanical Properties of Polymers in Biosurfaces: A Materials Science and Engineering Perspective; Wiley & Sons 2015
- T.G. Fox, and P.J. Flory, Journal of Applied Physics 21, 581–591 (1950)
- T. G. Fox and P. J. Flory, Journal of Polymer Science, 14, 315-319 (1954)